All about Medical Marijuana – Transportation Security Administration
Garota de Programa Ribeirão Preto - SP
Perfil
- Cidade: Ribeirão Preto - SP
- Eu Sou:
Apresentação:
All about Medical Marijuana – Transportation Security Administration
FDA considers a material to be “authorized for examination as a brand-new medication” if it is the subject of an Investigational New Medication application (IND) that has actually entered into impact. Under FDA’s guidelines (21 CFR 312. 2), unless a scientific investigation fulfills the restricted criteria in that policy, an IND is needed for all professional investigations of products that undergo section 505 of the FD&C Act.
Based on available evidence, FDA has actually ended that this is not the situation for THC or CBD. FDA is not conscious of any type of proof that would cast doubt on its existing final thoughts that THC and also CBD items are excluded from the nutritional supplement meaning under section 201(ff)( 3 )(B) of the FD&C Act.
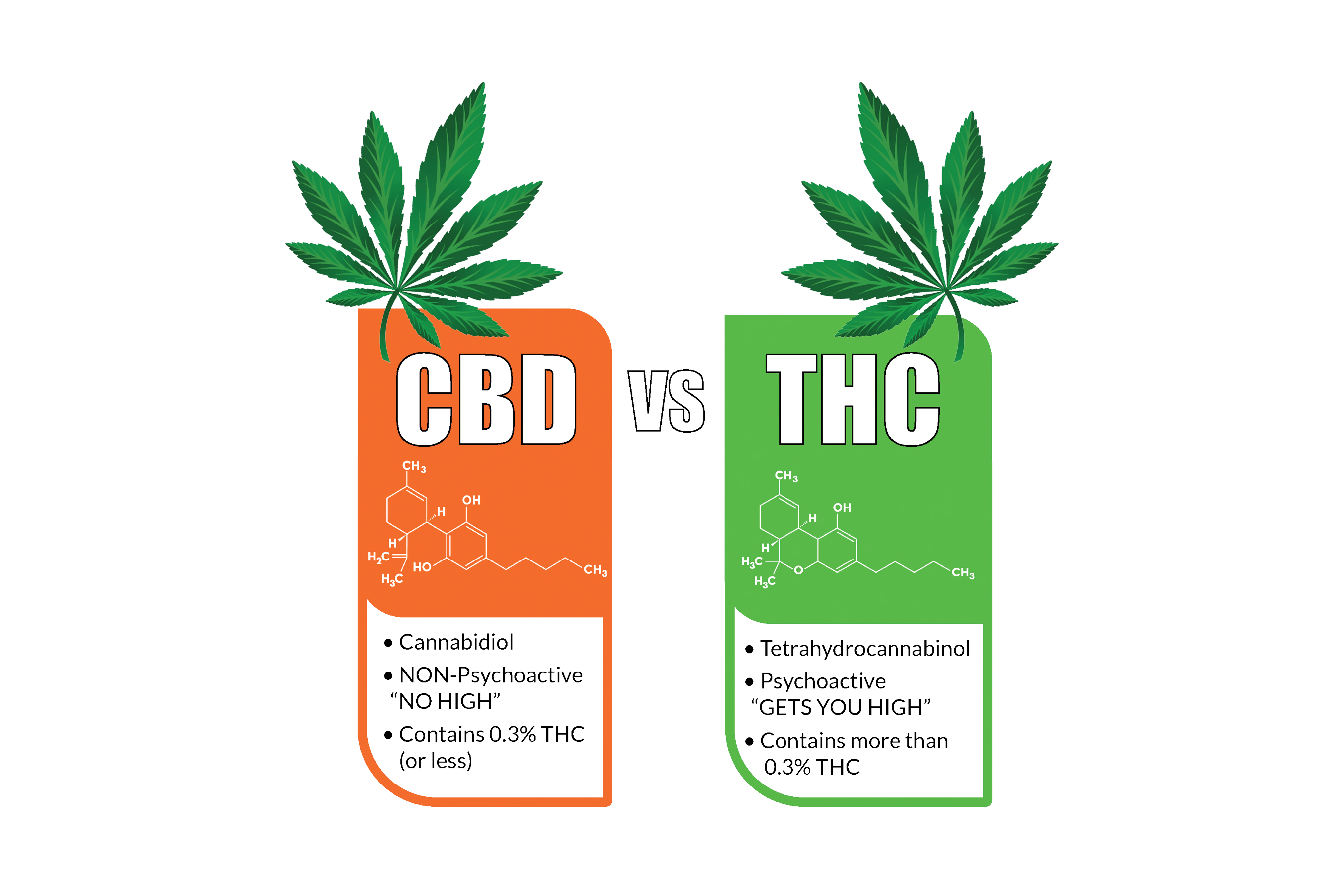
Components that are obtained from components https://wayofleaf.com/supplements/mushrooms/turkey-tail-mushrooms of the cannabis plant that do not include THC or CBD may fall outside the extent of this exclusion, and also consequently could be able to be marketed as nutritional supplements. Nonetheless, all products marketed as nutritional supplements have to abide with all applicable legislations and laws regulating dietary supplement items.
Not known Facts About Mary’s Medicinals: Lab Tested Thc And Cbd Products
355], or a medicine for which considerable clinical investigations have actually been set up and also for which the existence of such investigations has been revealed. There are exemptions, including when the medicine was marketed in food before the medicine was authorized or before the considerable clinical investigations involving the medication had been instituted or, when it comes to animal feed, that the medicine is a brand-new animal medicine authorized for usage in feed as well as utilized according to the approved labeling.
FDA has as a result concluded that it is a banned act to introduce or supply for introduction into interstate commerce any kind of food (including any pet food or feed) to which THC or CBD has actually been added. FDA is not familiar with any type of evidence that would certainly bring into question these conclusions. Interested events may present the firm with any proof that they assume has bearing on this issue.
When this legal restriction puts on a material, it prohibits the intro into interstate commerce of any food to which the material has actually been included unless FDA, in the company’s discretion, has actually provided a law approving the use of the material in the food (area 301(ll)( 2) of the FD&C Act [21 U.S.C.
Unknown Facts About The Farm Bill, Hemp Legalization And The Status Of Cbd
To day, no such guideline has been released for any compound. Active ingredients that are originated from components of the cannabis plant that do not include THC or CBD might fall outside the scope of 301(ll), and for that reason could be able to be contributed to food. As reviewed in Question # 12, specific hemp seed ingredients can be lawfully marketed in human food.
By law, any kind of compound deliberately included to food is a food additive, and also consequently subject to premarket evaluation and authorization by FDA, unless the material is normally recognized as safe (GRAS) by qualified professionals under the problems of its intended usage, or the use of the substance is or else excepted from the meaning of a food additive (areas 201(s) as well as 409 of the FD&C Act [ 21 U.S.C.
Other than the three hemp seed active ingredients pointed out concerned # 12, nothing else cannabis or cannabis-derived components have been the topic of an artificial additive petition, an examined GRAS notification, or have otherwise been authorized for use in food by FDA. Food firms that desire to make use of cannabis or cannabis-derived active ingredients in their foods are subject to the pertinent legislations and also policies that govern all food items, including those that connect to the food additive and GRAS procedures.
Getting My Cbd Vs. Thc: What’s The Difference? – Forbes Health To Work
These GRAS notifications associated just to making use of these active ingredients in human food. To date, FDA has actually not received any kind of GRAS notifications for using hemp-derived active ingredients in animal food (see Question # 25). Hemp seeds are the seeds of the Marijuana sativa plant. The seeds of the plant do not naturally include THC or CBD.
Consumption of these hemp seed-derived active ingredients is not efficient in making customers “high.” The GRAS final thoughts can apply to ingredients for human grocery store by other business, if they are made in such a way that follows the notices as well as they fulfill the detailed specs. Several of the designated uses for these components consist of adding them as resource of protein, carbohydrates, oil, and also various other nutrients to beverages (juices, healthy smoothies, healthy protein beverages, plant-based choices to dairy items), soups, dips, spreads, sauces, dressings, plant-based alternatives to meat items, treats, baked products, cereals, snacks and nourishment bars.

